產品介紹項目
LDPE Bottle Assembly
產品介紹:
伽瑪滅菌通風瓶組件,提供一個封閉的系統,以防止接種物轉移過程中的外部污染。通常用在細胞培養物轉移到生物反應器。
產品搜索:
規格訊息:

無菌接種物轉移至生物反應器

|
Materials of Construction |
||
|---|---|---|
|
Bottle |
LDPE |
|
|
Vent Filter |
Vent Membrane |
Hydrophobic PVDF |
|
Vent Body |
Polypropylene |
|
|
Connector |
Polypropylene |
|
|
Specifications |
||
|
Capacity |
500ml, 1 litre and 2 litre |
|
|
Vent Pore Size |
0.2μm |
|
|
Operational |
||
|
Max. Operating Temperature |
45 °C |
|
|
Sterilization |
Gamma irradiated @ 25kGy |
|
|
Assurance |
||
|
100% Integrity Tested |
Pressure Leak Test |
|
|
Bacterial Endotoxin |
Aqueous extracts exhibit < 0.25 EU/ml |
|
|
Fiber Release |
Passes test as per USP and comply with USFDA 21 CFR Part 210.3 (b) (6) for fiber release |
|
|
Particle Release |
Complies with USP <788> test for particulate matter in injection |
|
|
Endotoxin Testing |
Aqueous extract exhibit < 0.25 EU/ml as established by |
|
|
Sterility |
mdi LDPE vented bottle assemblies are sterilized by gamma irradiation to provide a sterility assurance level of 10-6. The sterilization process has been validated as per ISO 11137-2 which includes dose verification, dose mapping, and quarterly dose audits. The sterilization dose of 25 kGy has been substantiated through the careful definition of the test samples, bioburden testing of multiple lots of the selected test samples, calculation of verification dose, and sterility testing. |
|
|
Biosafety |
Passes Bioreactivity test, In-vivo, as per USP <88> for Class VI plastics |
|
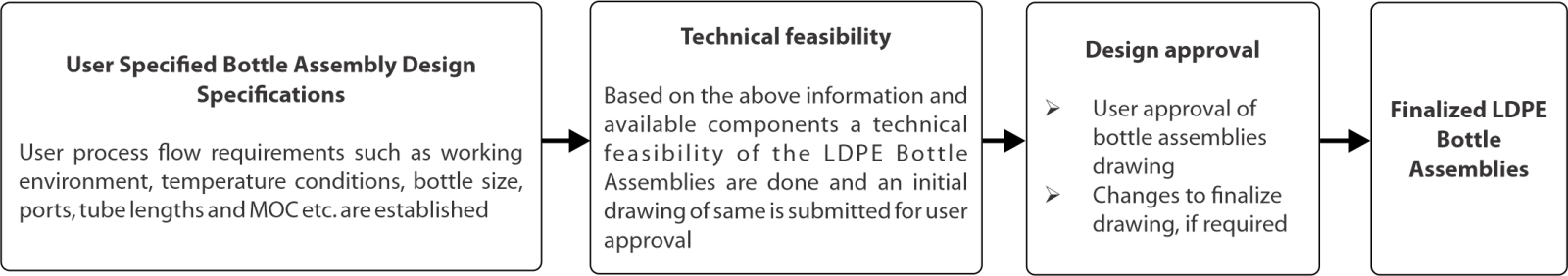
客製化
可根據使用者對管道、配件、端部連接、瓶子尺寸和連接器的要求進行客製化。
產品影片:
詢價車:
- Part NO.
- Qty
- Inquiry
- LDPE Bottle Assembly 客製化





